In the past 15 years, even as the number of new drug candidates in development nearly doubled, 90% of those that succeeded in preclinical animal studies failed in human trials. This high attrition rate – approximately 50% of failures for lack of efficacy and 30% for toxicity – raises the cost per new drug up to $2.8 billion according to recent estimates. In the current pandemic, mRNA vaccines went from virus sequence to first dosing in humans in an unprecedented 63 days – but the clinical trials will take more than a year. Even a single month saved in clinical trials would have meant trillions of dollars in global economic damage avoided.
For patients with organ failure, allograft donor transplant is often the only option. Worldwide, millions of patients on dialysis await a kidney transplant, for example. In the United States, alone, about 5,000 people die each year while waiting, making kidney disease a top ten killer – responsible for more deaths each year than breast cancer and more than $100B in Medicare costs. Of the more than 2 million patients with late-stage chronic kidney disease receiving dialysis replacement therapy, only 35% will survive the wait.
It’s time to bioengineer a change.
Indeed, if we are to build new solutions that catalyze a Health Age, we need 10x, scalable, transformations in the underlying time, cost, and efficiency of finding new solutions. Historically, fundamental advances in these parameters have exploded the number of innovators and the pace of innovation in fields as diverse as semiconductors, software, and genome sequencing.
Our selected performers.
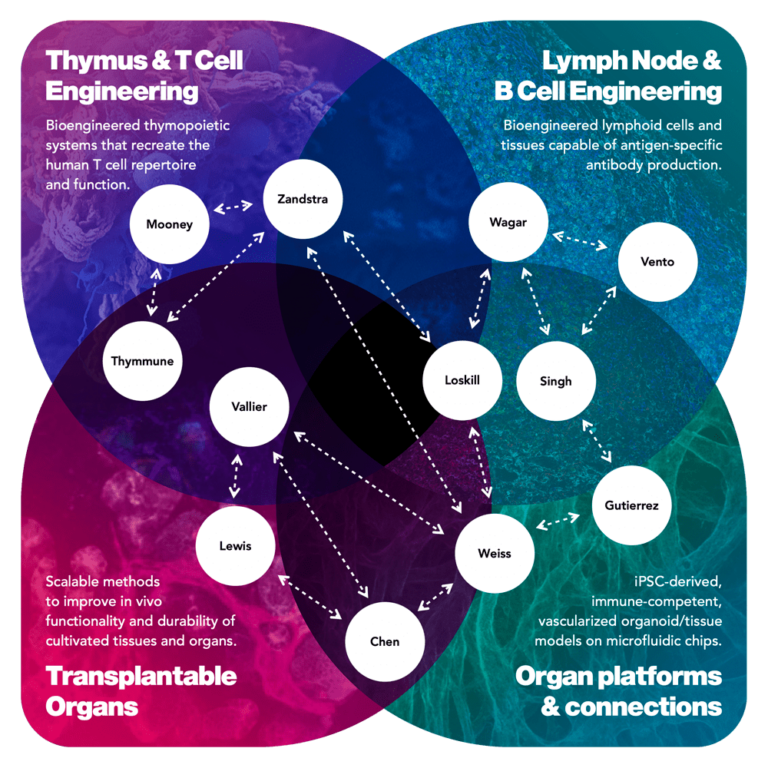
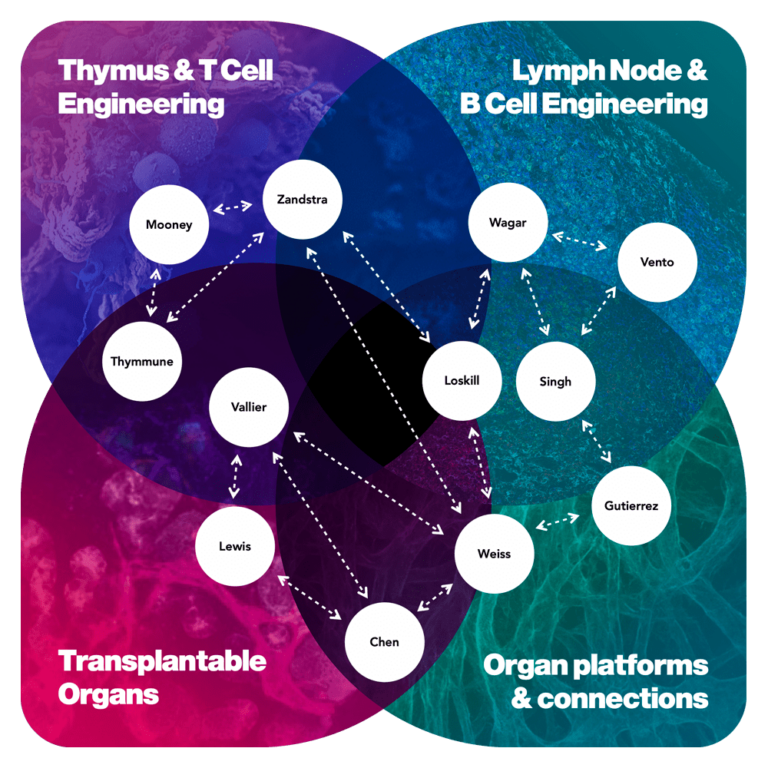
Team: Biola M. Javierre, Josep Carreras Leukaemia Research Institute; Leonardo Morsut, University of Southern California; Kyung-Ho Roh, The University of Alabama in Huntsville
Team: Biola M. Javierre, Josep Carreras Leukaemia Research Institute; Leonardo Morsut, University of Southern California; Kyung-Ho Roh, The University of Alabama in Huntsville
Engineering vascularized immunocompetent programmable organoids (VIP Organoids) for in vitro testing.
Engineering vascularized immunocompetent programmable organoids (VIP Organoids) for in vitro testing.
Building translational models of T cell-mediated immunity from human stem cells.
Building translational models of T cell-mediated immunity from human stem cells.
Peter Zandstra, University of British Columbia
Team: Megan Levings, University of British Columbia; Fabio Rossi, University of British Columbia; Geoffrey Schiebinger, University of British Columbia
Thymmune Therapeutics, Inc.
Team: Stan Wang, Thymmune Therapeutics, Inc.; Bing Lim, Thymmune Therapeutics, Inc.
Thymmune Therapeutics, Inc.
Team: Stan Wang, Thymmune Therapeutics, Inc.; Bing Lim, Thymmune Therapeutics, Inc.
Recreating the human TCR repertoire in vitro via engineering a functional human thymus.
Team: Rudolph Jaenisch, Massachusetts Institute of Technology; Lakshminarayanan Mahadevan, Harvard University
Recreating the human TCR repertoire in vitro via engineering a functional human thymus.
Team: Rudolph Jaenisch, Massachusetts Institute of Technology; Lakshminarayanan Mahadevan, Harvard University
Biomanufacturing vascularized kidney tissues for renal repair and replacement therapy.
Team: Leonardo Riella, Massachusetts General Hospital; Alice Chen, Trestle Biotherapeutics
Biomanufacturing vascularized kidney tissues for renal repair and replacement therapy.
Team: Leonardo Riella, Massachusetts General Hospital; Alice Chen, Trestle Biotherapeutics
Engineering artificial liver to restore hepatic function in acute and chronic liver diseases.
Team: Kourosh Saeb-Parsy, University of Cambridge; Kelly Stevens, University of Washington
Engineering artificial liver to restore hepatic function in acute and chronic liver diseases.
Team: Kourosh Saeb-Parsy, University of Cambridge; Kelly Stevens, University of Washington
Team: Julia Marzi, Eberhard Karls University Tübingen; Christian Schmees, Natural and Medical Sciences Institute at the University of Tübingen
Team: Julia Marzi, Eberhard Karls University Tübingen; Christian Schmees, Natural and Medical Sciences Institute at the University of Tübingen
Modelling lung health and diseases using stem cell-derived alveolar and innate immune cells on chip.
Modelling lung health and diseases using stem cell-derived alveolar and innate immune cells on chip.
Connecting tissues by engineering high density functional vascular, lymphatic, and ductal trees.
Team: Sangeeta N. Bhatia, Massachusetts Institute of Technology
Connecting tissues by engineering high density functional vascular, lymphatic, and ductal trees.
Team: Sangeeta N. Bhatia, Massachusetts Institute of Technology
Predicting immunogenicity using human tonsil organoids.
Predicting immunogenicity using human tonsil organoids.
Decoding specificity between antibodies and vaccines using integrated human lymphoid, gut and lung organoids.
Ankur Singh, Georgia Institute of Technology
Team: Jeremy Boss, Emory University; Ahmet Coskun, Georgia Institute of Technology; Andrés García, Georgia Institute of Technology; Krishnendu Roy, Georgia Institute of Technology
Decoding specificity between antibodies and vaccines using integrated human lymphoid, gut and lung organoids.
Ankur Singh, Georgia Institute of Technology
Team: Jeremy Boss, Emory University; Ahmet Coskun, Georgia Institute of Technology; Andrés García, Georgia Institute of Technology; Krishnendu Roy, Georgia Institute of Technology
Team: Biola M. Javierre, Josep Carreras Leukaemia Research Institute; Leonardo Morsut, University of Southern California; Kyung-Ho Roh, The University of Alabama in Huntsville
Team: Biola M. Javierre, Josep Carreras Leukaemia Research Institute; Leonardo Morsut, University of Southern California; Kyung-Ho Roh, The University of Alabama in Huntsville
Engineering vascularized immunocompetent programmable organoids (VIP Organoids) for in vitro testing.
Engineering vascularized immunocompetent programmable organoids (VIP Organoids) for in vitro testing.
Building translational models of T cell-mediated immunity from human stem cells.
Building translational models of T cell-mediated immunity from human stem cells.
Peter Zandstra, University of British Columbia
Team: Megan Levings, University of British Columbia; Fabio Rossi, University of British Columbia; Geoffrey Schiebinger, University of British Columbia
Thymmune Therapeutics, Inc.
Team: Stan Wang, Thymmune Therapeutics, Inc.; Bing Lim, Thymmune Therapeutics, Inc.
Thymmune Therapeutics, Inc.
Team: Stan Wang, Thymmune Therapeutics, Inc.; Bing Lim, Thymmune Therapeutics, Inc.
Recreating the human TCR repertoire in vitro via engineering a functional human thymus.
Team: Rudolph Jaenisch, Massachusetts Institute of Technology; Lakshminarayanan Mahadevan, Harvard University
Recreating the human TCR repertoire in vitro via engineering a functional human thymus.
Team: Rudolph Jaenisch, Massachusetts Institute of Technology; Lakshminarayanan Mahadevan, Harvard University
Biomanufacturing vascularized kidney tissues for renal repair and replacement therapy.
Team: Leonardo Riella, Massachusetts General Hospital; Alice Chen, Trestle Biotherapeutics
Biomanufacturing vascularized kidney tissues for renal repair and replacement therapy.
Team: Leonardo Riella, Massachusetts General Hospital; Alice Chen, Trestle Biotherapeutics
Engineering artificial liver to restore hepatic function in acute and chronic liver diseases.
Team: Kourosh Saeb-Parsy, University of Cambridge; Kelly Stevens, University of Washington
Engineering artificial liver to restore hepatic function in acute and chronic liver diseases.
Team: Kourosh Saeb-Parsy, University of Cambridge; Kelly Stevens, University of Washington
Team: Julia Marzi, Eberhard Karls University Tübingen; Christian Schmees, Natural and Medical Sciences Institute at the University of Tübingen
Team: Julia Marzi, Eberhard Karls University Tübingen; Christian Schmees, Natural and Medical Sciences Institute at the University of Tübingen
Modelling lung health and diseases using stem cell-derived alveolar and innate immune cells on chip.
Modelling lung health and diseases using stem cell-derived alveolar and innate immune cells on chip.
Connecting tissues by engineering high density functional vascular, lymphatic, and ductal trees.
Team: Sangeeta N. Bhatia, Massachusetts Institute of Technology
Connecting tissues by engineering high density functional vascular, lymphatic, and ductal trees.
Team: Sangeeta N. Bhatia, Massachusetts Institute of Technology
Predicting immunogenicity using human tonsil organoids.
Predicting immunogenicity using human tonsil organoids.
Decoding specificity between antibodies and vaccines using integrated human lymphoid, gut and lung organoids.
Ankur Singh, Georgia Institute of Technology
Team: Jeremy Boss, Emory University; Ahmet Coskun, Georgia Institute of Technology; Andrés García, Georgia Institute of Technology; Krishnendu Roy, Georgia Institute of Technology
Decoding specificity between antibodies and vaccines using integrated human lymphoid, gut and lung organoids.
Ankur Singh, Georgia Institute of Technology
Team: Jeremy Boss, Emory University; Ahmet Coskun, Georgia Institute of Technology; Andrés García, Georgia Institute of Technology; Krishnendu Roy, Georgia Institute of Technology
Program goals.
In this program, we aim to leverage the power of bioengineering to advance stem cells, organoids, and whole organ systems and connections that recapitulate human physiology in vitro and restore vital functions in vivo. We have two goals:
1. Bioengineer a multiorgan platform that recreates human immunological responses with sufficient fidelity to double the predictive value of a preclinical trial with respect to efficacy, toxicity and immunogenicity for therapeutic interventions targeting cancer, autoimmune and infectious diseases.
1a. The platform should represent tissue-resident and lymphatic immune systems and recreate immunological mechanisms involved in pathologies and drug-induced reactions (including effects on target and non-target organs), as demonstrated in retrospective studies of a statistically relevant number of immunomodulator drugs.
1b. The platform should predict the wanted immunogenic activity of vaccines and the unwanted immunogenic risks of therapeutics, especially to inform the design principles for an immunologically tolerated transplantable organ.
2. Demonstrate the advances necessary to restore organ functions using cultivated organs or biological/synthetic hybrid systems that would result in a doubling of the 5-year survival rate of patients on replacement therapy or awaiting organ transplantation and point to a fully transplantable, non-rejected, human organ within 10 years.
2a. In the program we expect to produce the first hypo-immunogenic living functional unit at human scale, for example a kidney nephron with a filtration rate at least equivalent to moderate CKD stage and an immunogenicity profile in the range of an autologous graft.
2b. Advances are expected to contribute to the physiological relevance and robustness of organ system platforms for in vitro applications such as drug testing.
Program Director.
Annie Moisan, PhD. Annie’s expertise is in the mechanistic biology of drug targets and preclinical safety strategies of novel therapeutics. She has worked at Idorsia Pharmaceuticals, Roche Innovation Center Basel, and as a postdoctoral fellow in genetics and stem cell biology at Harvard Medical School. She earned her Ph.D. in molecular and cell biology from University of Sherbrooke, Canada.
Who are eligible Wellcome Leap program performers?
Performers are from universities and research institutions: small, medium and large companies (including venture-backed); and government or non-profit research organizations. We encourage individuals, research labs, companies, or small teams to apply in program areas best aligned with their expertise and capabilities. It is not necessary to form a large consortium or a single team to address all thrusts or an entire program goal in an abstract or proposal. Indeed, one of the benefits of our programs is that we actively facilitate collaboration and synergies dynamically among performers as we make progress together toward the program’s goals.
Program announcement.
15-Day Abstract review round.
30-Day Full proposal review round.